Riding the wave of innovation How to secure four FDA clearances and two FDA Breakthrough Designations in four years
- | By Ultromics
- Articles, Heart Failure
Ross Upton PhD, Founder, CEO and Chief Scientific Officer, Ultromics, and AI Co-Chair, HeartShare, NIH in conversation with Gary Woodward PhD, Chief Product Officer, Ultromics.
With technology, regulation, and clinical need all in a constant state of flux, AI diagnostics is a fast moving field. At Ultromics, we have found that success relies on staying ahead of the curve, being ready and able to pivot as the landscape shifts beneath you – and being dedicated to a shared ambition.
Our chief product officer (CPO), Gary Woodward, and I first met in 2011, a chance encounter in a coffee shop on the University of Surrey campus as students. Our friendship continued when we both landed in Oxford on clinical scientist placements, Gary in clinical biochemistry and me in cardiovascular imaging, and started talking about the potential of AI. Those early conversations led to us working side-by-side, building Ultromics’ AI-based echocardiography solutions, and, in the last four years alone, securing four FDA clearances and two FDA breakthrough designations.
In a rare quiet five minutes, I asked Gary to outline our journey so far, share the secrets of our success, and tell me where we – and the cardiovascular field as a whole – are headed next. Here’s what he had to say.
Tell us about your current role, and the journey you took to get here?
As CPO, my days are a blend of science and strategy. I oversee the technical development of AI products, nurturing those early sparks of innovation until they are ready to hit the market and make a real difference. I also work with Ross who doubles up as our Chief Scientific Officer, and his team to generate the scientific evidence needed to back our technology, ensure our products align with market access strategies, manage our intellectual property, and handle our product portfolio. Finally, I work with Amir Hasan, our chief operating officer, on strategic collaborations and the pharma solutions side of the business.
My journey has taken me across numerous facets of healthcare and scientific research. I cut my scientific teeth in the consumer health division of GlaxoSmithKline, as part of my PhD in biochemistry. Then came my Masters, HCPC State Registration in Clinical Science, and my association with the Royal College of Pathologists.
Before moving into the startup world, I was on the ground, running diagnostics at the University College London (UCL), Oxford University Hospitals and University Hospitals Birmingham, among others. As part of a medical multidisciplinary team specializing in rare disease at UCL, it was my job to embed new diagnostics into clinical practice and trials alongside day-to-day clinical biochemistry.
I started using AI in my own practice, developing machine learning models for steroid and amino acid analysis, after talking with Ross when we were both in Oxford. I quickly realised the huge potential of this technology, and moved to an imaging-based start-up to hone my skills before taking on my role at Ultromics.
How have regulations around AI in healthcare evolved over the six years you have been working in the area?
FDA AI regulation is a dynamic landscape. My curiosity around AI diagnostics started to blossom in the early 2010s, and by the middle of that decade the FDA had started to approve AI-driven medical devices. A few years later, the body had released an AI/ML discussion paper and an action plan, signalling the beginnings of a robust, transparent framework for the technology's future.
One of the biggest evolutions has been an increase in the transparency and explainability of AI, overcoming the “black box” paradox. It's one thing for AI to make decisions, but another to unpack the 'how' and 'why' for clinicians and patients. We're still on the cusp here, and more comprehensive guidance is needed, but it's thrilling to be part of this pioneering work.
How have you navigated the evolving regulations?
Engaging proactively with the FDA and maintaining open communication channels are critical to ensuring we comply with current standards, and are well positioned to adapt to future changes. The FDA’s increasing demand for greater detail on AI development, human factors testing and cybersecurity, alongside multiple recent guidance updates for AI in medical devices and the introduction of the pilot Total Product Life Cycle Advisory Program (TAP), have underscored the importance of comprehensive regulatory planning.
EchoGo® Pro, our predicate device that uses AI to analyse stress echocardiograms and assess the possibility of significant coronary artery disease (CAD), was an exemplar of how to develop AI medical devices in line with regulatory guidance. Launched in 2020, it was the first “AI EchoDx” to reach the market. It was simple in principle, and used imaging biomarkers that are explainable and comparable to regional and temporal anatomy and pathology. The clinical impact was assessed, to demonstrate how it could deliver improvements in clinical decision making, and it is now in the final stages of a first of its kind multi-site prospective randomized control trial, funded by the UK government.
Next came EchoGo® Core. Launched in 2021, the software was designed to measure cardiac function. This enabled us to publish several papers demonstrating that AI-based measurements were better correlated to patient outcomes than human-derived measurements.
EchoGo® Heart Failure, our 2022 entrant, really epitomizes our approach. Awarded breakthrough status by the FDA, it's a sophisticated tool in the battle against heart failure with preserved ejection fraction (HFpEF). Its full automation and lack of a user interface allows for an unbiased, AI-driven diagnosis. has already shown that it can discriminate between people with and without HFpEF more often than clinical scores, and we are presenting more exciting findings at the Technology and Heart Failure Therapeutics (THT) 2024 conference later this week. In essence, it represents a huge leap forward both in the technology and in clinical management.
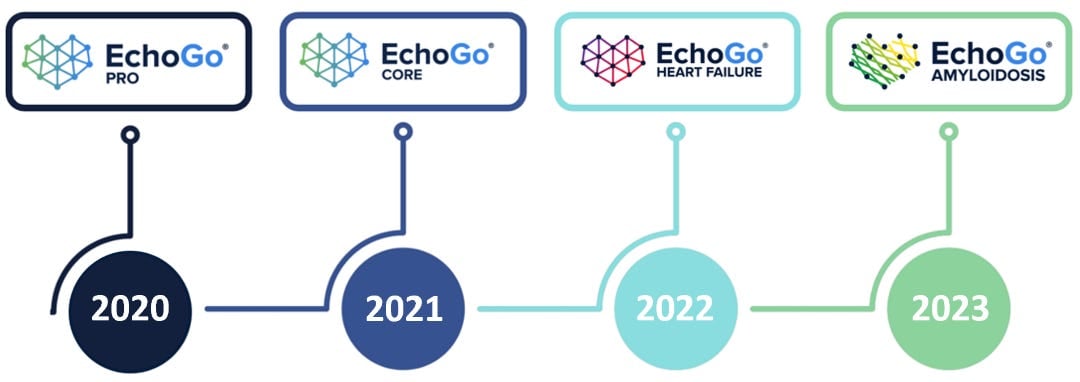
EchoGo® Amyloidosis pending 510(k) clearance
The latest addition to our portfolio is EchoGo® Amyloidosis 1.0. Another FDA breakthrough device, it has been accepted into the TAP program, making it one of a select group of innovations shaping the future of healthcare technology. The tool, which takes an apical four-chamber cine loop and delivers a binary classification indicative of the presence or absence of cardiac amyloidosis, has been meticulously trained and validated using independent datasets.
Throughout this journey, my mantra has been ‘clarity, transparency and collaboration’. Working hand-in-hand with clinicians, academics and regulatory bodies, we've not just kept pace with AI's rapid evolution, but often stayed a step ahead.
How has Ultromics secured an FDA clearance every year for the last four years?
The race to market is not just about speed, but strategic foresight and the ability to swiftly adapt to changing regulations and clinical needs. Ongoing engagement with regulators, keeping up to date with the clinical landscape, and smart trial design for evidence generation can greatly improve timelines. This allows us to meet FDA standards for safety and efficacy, and to adapt our products based on emerging data and feedback.
EchoGo® Pro, for example, was developed to analyze stress echos and detect significant CAD, but, as we neared launch, medical guidelines began advocating for CT angiography over stress echo. Around the same time, cardiac strain measurements by echo became a hot topic, securing support from the professional associations, CMS and clinical guidelines. This presented us with an opportunity to leverage EchoGo® Pro’s functionality to create EchoGo® Core, which automates the measurement of key cardiac parameters – including strain- which helps in the assessment of Heart Failure.
This set the foundation for our next AI EchoDx, which focused on heart failure with preserved ejection fraction (HFpEF) and, as we moved into the space, the clinical landscape moved with us. In 2021, a therapeutic breakthrough demonstrated that SGLT2 inhibitors reduced the risk of hospitalization for patients suffering with HFpEF. Diagnosis of the condition, however, remained significantly challenging. This presented us with a huge opportunity to help tackle an unmet medical need, so we accelerated the development of EchoGo® Heart Failure.
How does Ultromics’ US market authorisation portfolio compare to others in the space?
FDA-cleared AI algorithms in the cardiovascular space make up 16% of total approvals, with Ultromics holding three cleared products comprising four algorithms. This positions us alongside leading MedTech companies like Medtronic, Edwards Life Sciences, and GE Healthcare.
FDA clearance, however, is just the start line. It does not guarantee widespread adoption, and the post-approval journey, which includes refining the technology to meet the practical demands of healthcare providers, is just as important as the pre-approval one. 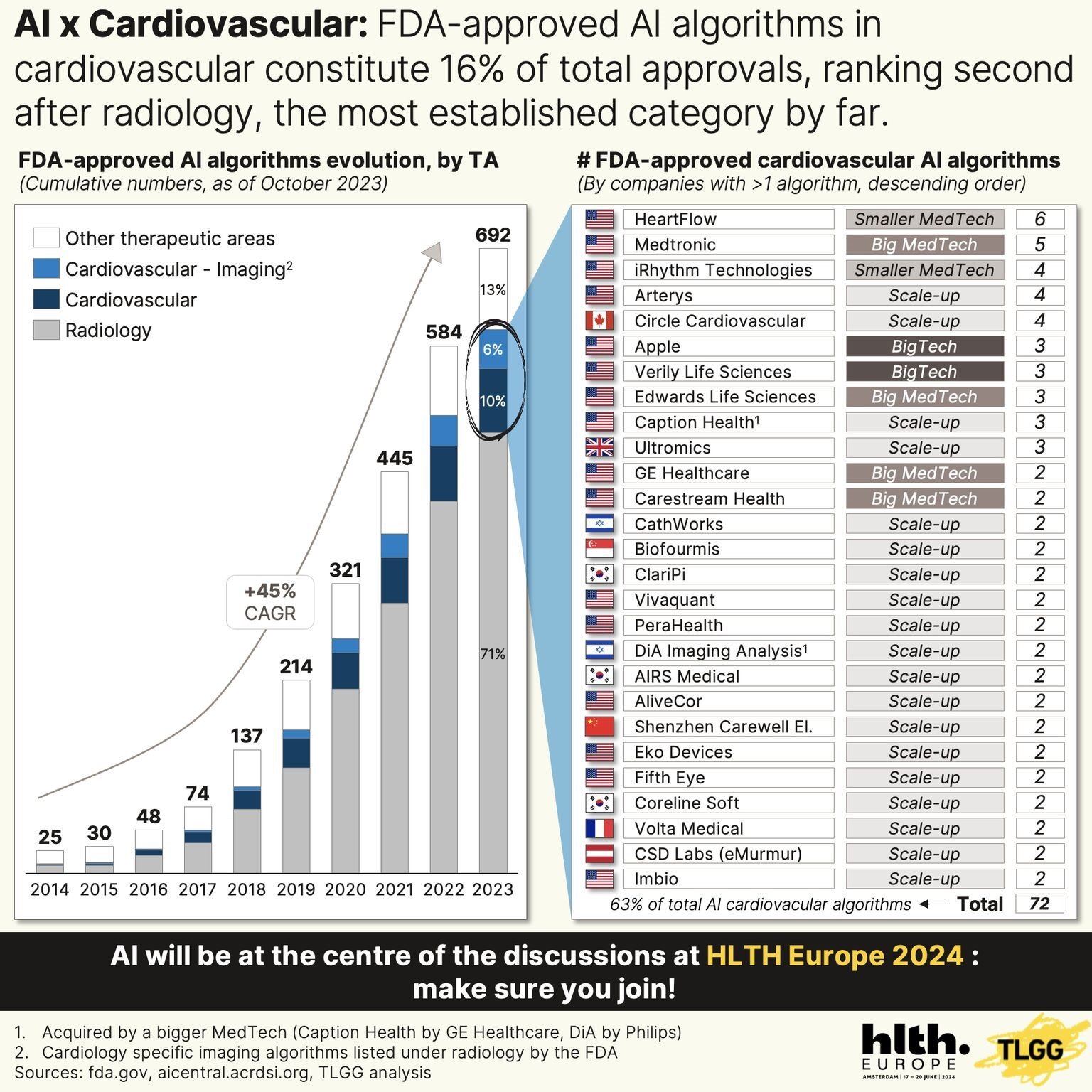
Figure Reference: FDA-approved AI algorithms in the cardiovascular space make up 16% of total approvals
What’s next for AI-driven echocardiography?
There is a marked interest in cardiac amyloidosis as a devastating disease that causes acute heart failure and is not as rare as first thought. Numerous therapeutic organizations have made notable progress in this field, and are poised to introduce therapies that address the underlying mechanisms of the disease. This, combined with the FDA’s intention to reclassify most class II (high risk) in vitro diagnostic devices (IVDs) as class II (moderate risk), represents a unique opportunity for AI-driven echo.
The FDA’s shift will streamline the regulatory pathway, allowing manufacturers to pursue marketing clearance through the less burdensome premarket notification (510(k)) pathway, rather than the premarket approval (PMA) route. For cardiac amyloidosis, where diagnostic precision is crucial for the effective use of emerging therapies, this evolution could accelerate the development and accessibility of companion-like diagnostics.
With all this in mind, Ultromics is actively developing EchoGo® Amyloidosis, a screening tool that aims to leverage the profound impact of early and accurate diagnosis on treatment outcomes and patient care. By repurposing EchoGo® Heart Failure’s underlying software, we can swiftly adapt the technology to cardiac amyloidosis screening, efficiently meeting the emerging needs of the healthcare market, pharma interests, and shifting regulatory landscape. To accelerate our progress, we have partnered with industry leaders such as Johnson & Johnson Innovative Medicine and Pfizer. This is not a move we make alone. Indeed, several medical device companies have emerged to join the fight against cardiac amyloidosis. Organizations including Anumana, Invision AI, Ensight-AI, Viz AI, iCardio.AI and Atman Health have launched technologies across a range of modalities, including echocardiography, electrocardiography, and electronic medical record screening. Together, they create a potentially symbiotic, comprehensive ecosystem of technologies that could help address the challenges of detecting and managing cardiac amyloidosis from multiple angles, and multiple points of patient entry.
By harnessing the strengths of AI, predictive analytics, and personalized health technologies, we are setting the stage for a future where this condition can be identified and treated much earlier in the disease course.
The future of AI-driven EchoDx is bright. It is up to us, as innovators, to work hand in hand with clinicians, regulators, and other stakeholders to fulfil its potential.
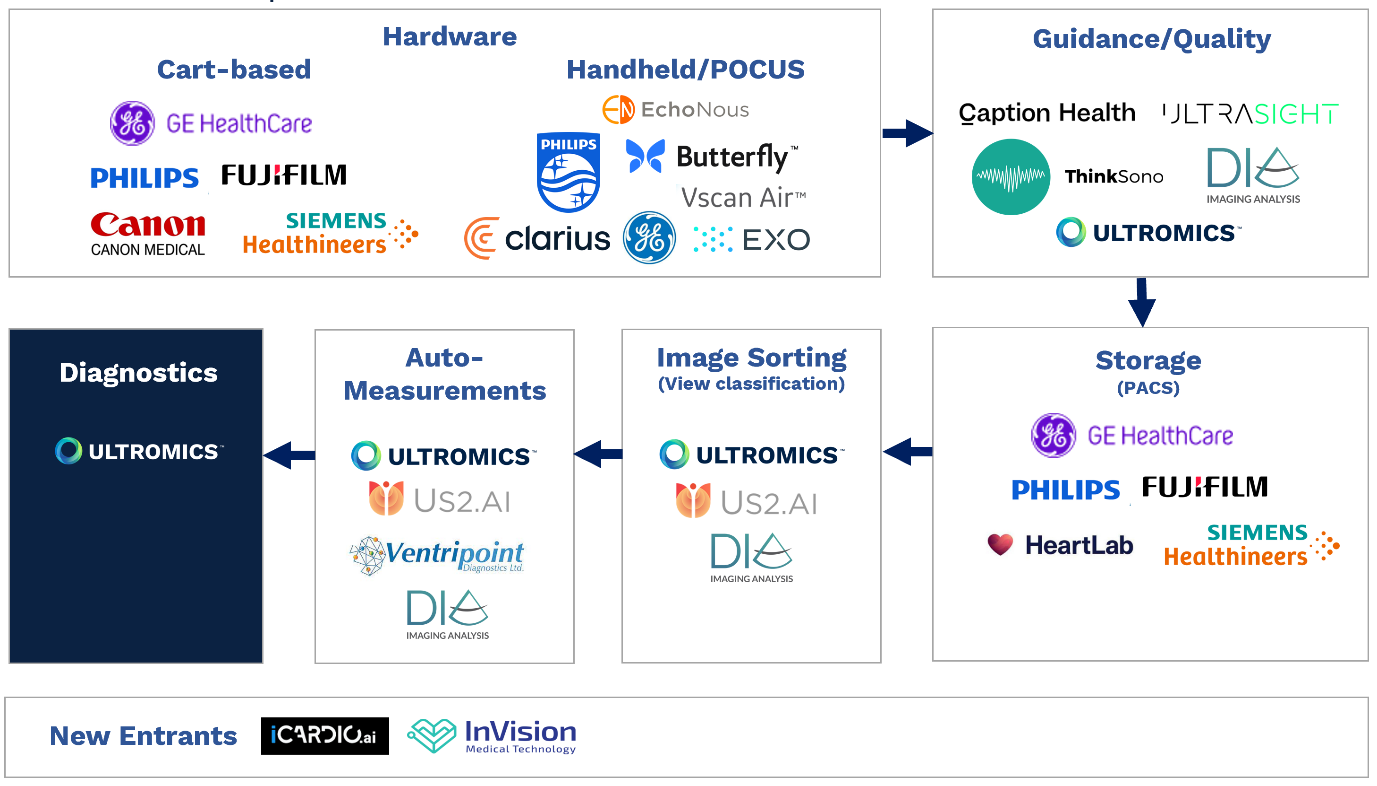
Echocardiography AI market diagram
Curious about upcoming research and innovation?
Sign up to hear about the latest news.

