
Cardiac Amyloidosis Uncovered
- The first FDA-cleared, AI-based screening tool designed to help identify patients at risk of cardiac amyloidosis from an echocardiogram.

EchoGo® Amyloidosis
Designated as a Breakthrough Device by the FDA, EchoGo® Amyloidosis is a standalone, AI-enabled screening tool that assists in the identification of cardiac amyloidosis during routine cardiovascular assessments. It analyzes echocardiographic data and provides insights that support physicians in identifying at-risk patients.

Proven to Detect Amyloidosis with Precision
EchoGo® Amyloidosis analyzes echocardiographic images to support early identification of patients who may require further evaluation for cardiac amyloidosis. By facilitating systematic screening, EchoGo® Amyloidosis helps prioritize follow-up and improve care pathways.
<BR>
Early detection is critical
Cardiac amyloidosis is progressive and often misdiagnosed—frequently mistaken for HFpEF. Delayed identification can lead to advanced heart failure and worse outcomes.
Early detection enables timely treatment, including stabilizers, gene-silencing therapies, or chemotherapy.
While echocardiography is a key first-line tool, subtle signs of amyloidosis are often missed, even by experienced clinicians.
EchoGo® Amyloidosis supports early, systematic identification—helping clinicians recognize at-risk patients sooner and streamline referrals.
5+ MD visits before diagnosis (ATTR)3
14% of HF population with Amyloid4
65% 5-year mortality (AL)5
52% 90-day readmission6
Data-Driven Innovation
“Novel AI-based diagnostic tools such as EchoGo® Amyloidosis from Ultromics should help facilitate disease identification, particularly in clinics and hospitals restricted by expertise and resource. ”
Research and Partnerships


Tested and Proven By Leading Cardioloists
Seamless Integration with EchoGo® Platform
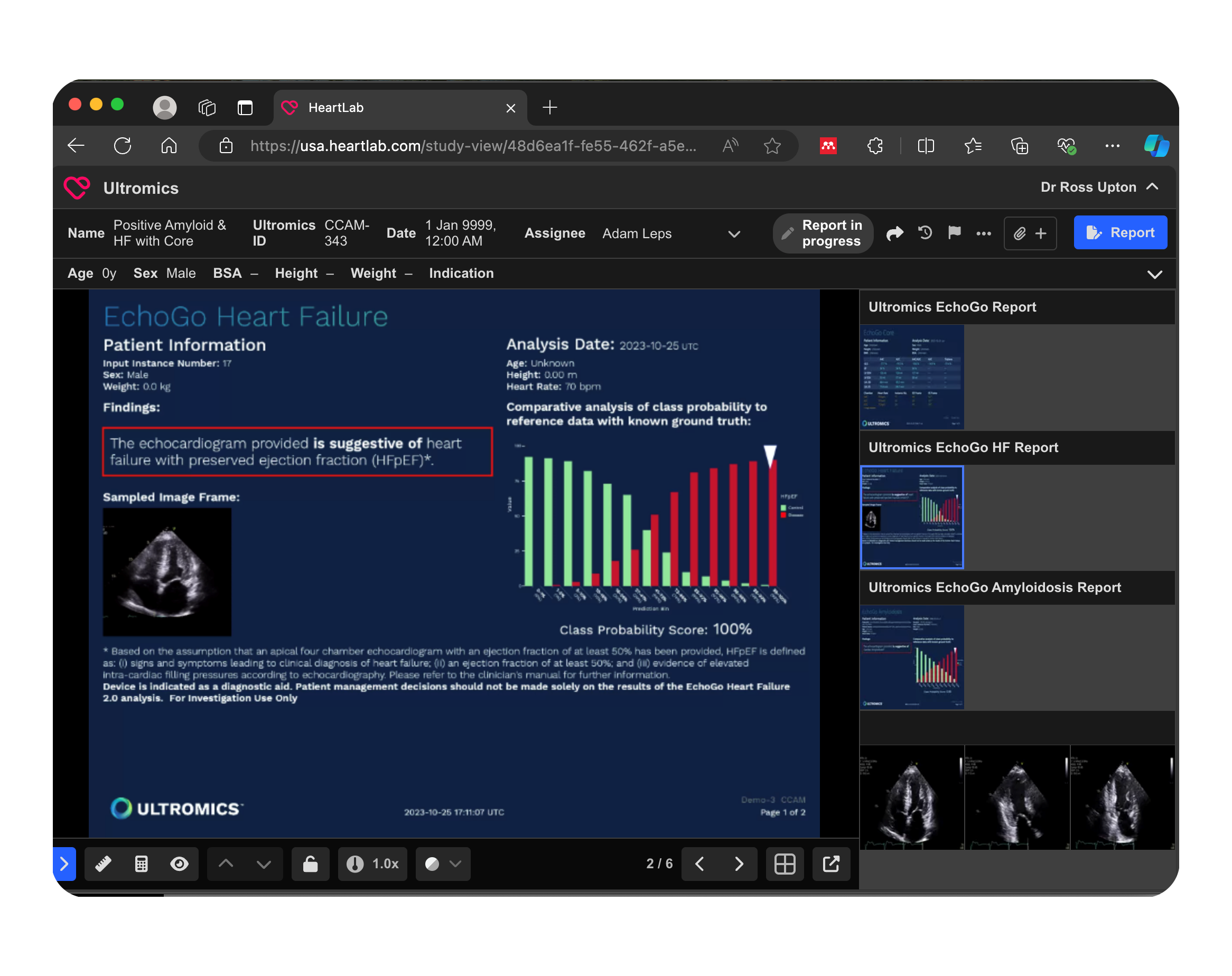
The EchoGo® platform operates seamlessly in the background, running Ultromics’ advanced algorithms at scale. Designed to integrate effortlessly into your workflow, EchoGo® connects with your existing IT infrastructure and provides seamless access to reports.
Integrate Seamlessly
- Easily implemented, with no hardware/software at site.
- Integrates with existing workflows and IT stacks.
- Secure cloud VPN connection and data anonymized.
Analyze Data
- EchoGo® analyzes data, identifies suspected findings, and generates a report.
- A report is sent to PACS for the interpreting physician.
- Operates in an automated DICOM workflow.
Get Reimbursed*
- Inpatient: NTAP Code ICD-10 XXE2X19 – $1,000 per analysis (Medicare-covered).
- Outpatient: HCPCS Code C9786, Clinical APC Code 574 – $285 per analysis (covered by Medicare and select commercial insurers).
-
EchoGo® Amyloidosis operates in tandem with EchoGo® Heart Failure which is eligible for reimbursement.*
Scale with Ease
- Deploy Ultromics’ AI algorithms across any care setting—from hospitals to clinics—without workflow disruption.
- Minimal training to onboard new staff with ease.
AI-Driven Cardiology Diagnostics
This integrated PACS solution leverages AI-powered analysis to suggest heart failure classifications directly from echocardiogram data. The automated reporting within PACS allows clinicians to view diagnostic insights directly in the workflow. This streamlined approach supports faster, data-driven clinical decisions.
QC + secured
-
CyberEssentials, ISO 27001, and ISO 13485
-
HIPAA Compliant
Vendor Neutral
-
Standard interfaces into all PACS
-
Secure, encrypted VPN
Cloud Native
-
Optimal resource savings, with no new hardware or software required
-
No need to learn or train on additional systems
Dashboard Analytics
-
CyberEssentials, ISO 27001, and ISO 13485
-
HIPAA Compliant
Secure by design
EchoGo® is a secure SaaS model. It complies with all relevant privacy and security regulations, including HIPPA and SOC 2 Type 2. Data is anonymized, encrypted in transit and at rest, ensuring patient confidentiality at all times.








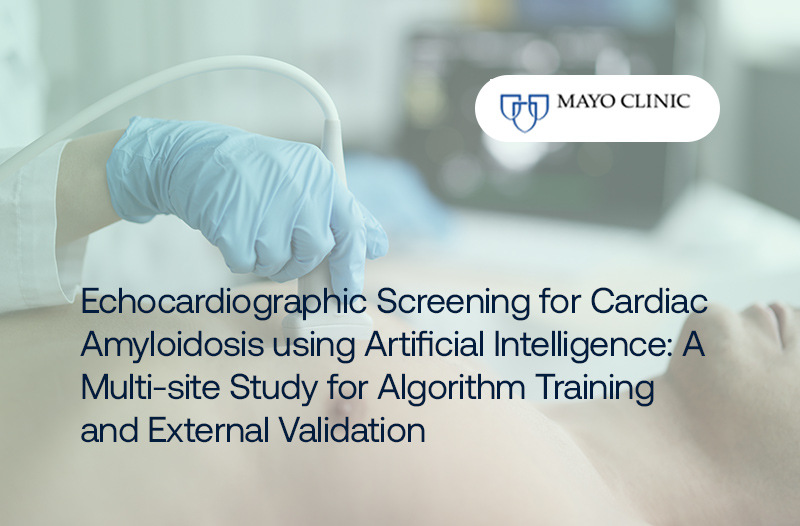
Latest Research
In partnership with Amyloidosis specialists at Mayo Clinic, our research investigates the diagnostic performance of EchoGo® Amyloidosis in a clinical setting. Using a robust testing data set of 3,000 cases, EchoGo® demonstrated a high diagnostic accuracy:
0.93 AUC
84% Sensitivity
92% Specificity

References:
- Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. European heart journal. 2018 Aug;39(30):2799–806.
- Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Mar;30(9):989–95.
- Gertz M, Adams D, Ando Y, Beirão JM, Bokhari S, Coelho T, et al. Avoiding misdiagnosis: expert consensus recommendations for the suspicion and diagnosis of transthyretin amyloidosis for the general practitioner. BMC Family Practice. 2020 Sep 23;21(1).
- Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, et al. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart failure. 2020 Sep 1;8(9):712–24.
- Escher F, Senoner M, Doerler J, Zaruba MM, Messner M, Mussner-Seeber C, et al. When and how do patients with cardiac amyloidosis die? Clinical Research in Cardiology. 2019 May 27;109(1):78–88.
- Berthelot E, Amaury Broussier, Hittinger L, Donadio C, Rovani X, Salengro E, et al. Patients with cardiac amyloidosis are at a greater risk of mortality and hospital readmission after acute heart failure. ESC heart failure. 2023 Apr 13;10(3):2042–50.
Discover the full potential of EchoGo®
Access both HFpEF and Amyloidosis in one seamless platform.


