
Global longitudinal strain can improve the characterization of Stage A and B heart failure
- | By Ultromics
- Articles, Heart Failure
Full paper published here: https://www.jacc.org/doi/10.1016/j.jcmg.2022.03.007
The association between global longitudinal strain (GLS) and clinical outcomes of heart failure (HF) is well established. The American College of Cardiology and other major cardiovascular societies recommend GLS for heart failure patients[1], with numerous studies proving its prognostic potential.
The European Society of Cardiology (ESC) heart failure guidelines, published in December 2021, suggested “GLS is an early indicator of HF and adverse outcomes”. The AHA/ACC/HFSA HF guidelines also suggest a GLS<16% indicates abnormal left ventricular filling pressures, so there is even more importance placed upon adding GLS measurements to regular reporting practices.
According to literature published this month in JACC Cardiovascular Imaging “Improving the Characterization of Stage A and B Heart Failure by Adding Global Longitudinal Strain”, GLS is a significant and important prognostic indicator in the prediction of heart failure.
Background
Current guidelines distinguish stage B heart failure (SBHF) (asymptomatic left ventricular [LV] dysfunction) from stage A heart failure (SAHF) (asymptomatic with heart failure [HF] risk factors) on the basis of myocardial infarction, LV remodeling (hypertrophy or reduced ejection fraction [EF]) or valvular disease. However, subclinical HF with preserved EF may not be identified with these criteria.
Objectives
The purpose of this study was to assess the prediction of incident HF with global longitudinal strain (GLS) in patients with SAHF and SBHF.
Methods
The authors analyzed echocardiograms (including GLS) in 447 patients (age 65 ± 11 years; 77% male) enrolled in a prospective study of HF in individuals at risk of incident HF, with normal or mildly impaired EF (≥40%). Long-term follow-up was obtained via data linkage. Analysis was performed using a competing risks model.
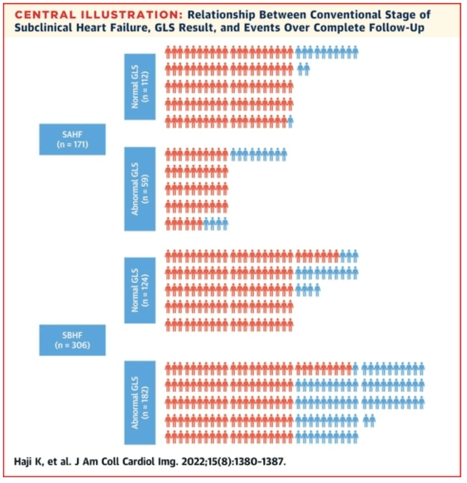
Results
After a median of 9 years of follow-up, 50 (10%) of the 447 patients had new HF admissions, and 87 (18%) died. In multivariable analysis, all imaging variables were independent predictors of HF admissions, including left ventricular ejection fraction (LVEF) (HR: 0.97 [95% CI: 0.94-0.99]), LV mass index (HR: 1.01 [95% CI: 1.00-1.02]), left atrial volume index (HR: 1.02 [95% CI: 1.00-1.05]), and E/e′ (HR: 1.05 [95% CI: 1.01-1.24]), incremental to clinical variables (age and Charlson comorbidity score). However, the addition of GLS provided value incremental to both clinical and other echocardiographic parameters (P = 0.004). Impaired GLS (<18%) (HR: 4.09 [95% CI: 1.87-8.92]) was independent and incremental to all clinical and other echocardiographic variables in predicting HF, and impaired LVEF, left ventricular hypertrophy, left atrial enlargement, high E/e′, or SBHF were not predictive.
Conclusions
The inclusion of GLS as a criterion for SBHF would add independent and incremental information to standard markers of SBHF for the prediction of subsequent HF admissions.
- GLS was an independent predictor of first heart failure admission in patients with classically defined SAHF and SBHF, incremental to the established clinical risk factors and conventional echocardiographic characteristics.
- GLS was shown to provide more value than LVEF and other echocardiographic parameters in the identification of subclinical heart failure.
- Only impaired GLS was independent and incremental to all clinical and other echocardiographic variables in predicting HF, where impaired LVEF, left ventricular hypertrophy, left atrial enlargement, high E/e′, or SBHF were not predictive.
- The risk of developing HF increased with addition of abnormal GLS with or without stage B heart failure.
- GLS was the only imaging variable that increased the prediction of HF hospitalization and death with the combination of clinical variables.
- The addition of conventional assessment of SBHF did not improve the prediction provided by clinical variable and GLS
How Ultromics Helps Detect Heart Failure
Ultromics is an AI-powered platform which can be connected through the cloud to automate LV insights, and provide reports whenever an echocardiogram is performed. The technology offers significantly improved heart failure detection by automating ejection fraction (EF), volumes, and –notably – global longitudinal strain (GLS), with zero variability.
Related: See the latest study results showing how Ultromics can outperform manual operators at predicting heart failure and accurately correlates to a known outcome.
Ultromics' revolutionary use of AI and cloud capabilities can automate GLS and EF with speed, accuracy and zero variability. The platform replaces legacy on-premises software, so clinicians can benefit from streamlined care which holds greater flexibility and accelerated innovation.
Full paper published here: https://www.jacc.org/doi/10.1016/j.jcmg.2022.03.007
Curious about upcoming research and innovation?
Sign up to hear about the latest news.

