Cellular and molecular differences between HFpEF and HFrEF
- | By Ultromics
- Articles, Heart Failure
Heart failure affects about 6.2 million Americans and is rising in prevalence. With half of these cases presenting as heart failure with preserved ejection fraction (HFpEF), it’s essential for both the medical community and the population at large to have a better understanding of its pathology. [1,2]
Knowing the differences between HFpEF and its counterpart, heart failure with reduced ejection fraction (HFrEF), including contrasts in their cellular and molecular presentations, can be key to gaining an increased understanding of this complex syndrome and may lead to improved therapeutic options.
Structural, Cellular, and Molecular Considerations in the Development of HFrEF and HFpEF
HFpEF, defined by an ejection fraction (EF) of greater than 50%, is caused by structural and cellular alterations in the heart that include cardiomyocyte hypertrophy, fibrosis, and inflammation. These eventually lead to diastolic dysfunction and an inability of the left ventricle to relax properly. [4]
In contrast, HFrEF, defined by an EF of less than 40%, is characterized by structural alterations and substantial cardiomyocyte loss. This leads to the development of systolic dysfunction, or the ability of the left ventricle to contract properly. [4]
Heart failure with mid-range or mildly reduced EF (HFmrEF), is an
intermediate stage, with an EF between 40–49%, that generally progresses either to HFpEF (25% of cases) or HFrEF (33% of cases). With regard to ischaemic aetiology, HFmrEF more resembles HFrEF, but HFmrEF has a higher frequency of underlying coronary artery disease (CAD) and a better overall prognosis.
Comorbidities
While there are differences between the cellular and structural comorbidities of HFpEF and HFrEF, there are some similarities. Endothelial cells, for example, are present in both HFpEF and HFrEF as shown by a similar loss in cardiac microvascular density. [4]
This, however, is where the similarities end. Contrasting comorbidities in HFpEF and HFrEF can affect endothelial function in different ways, with microvascular complications being more prevalent in HFpEF than in HFrEF [4].
Endothelial dysfunction also occurs much earlier in the progression of HFpEF, which means it can be used as an early marker of the condition. Contrastingly, it tends to be a late-stage symptom in HFrEF. [4]
As mentioned, inflammation also plays a key role in the different molecular comorbidities leading to HFpEF and HFrEF.
Sterile inflammation, for example, generally occurs in patients who go on to develop HFrEF. This type of inflammation results from acute post-ischemic or toxic necrosis, massive trauma, hemorrhage, and/or resuscitation. [4]
Non-sterile inflammation resulting from viral infections, such as viral myocarditis, can also lead to HFrEF. This type of infection triggers an acute phase of inflammation that can lead to scar tissue and reduced contractile function in the chronic phase that all lead to HFrEF. [4]
For HFpEF patients, it’s the low-grade chronic systemic inflammation that plays a key role in microvascular changes. This type of inflammation specifically results from noncardiac metabolic risk factors, including obesity, diabetes, anemia, hypertension, chronic obstructive pulmonary disease (COPD), auto-immune diseases, and renal insufficiency. [4]
Other Key Differences Between HFpEF and HFrEF: Risk Factors, Diagnosis, and Treatment
Risk factors
Molecular and cellular differences between HFpEF and HFrEF suggest that risk factors between the two conditions can also vary. Patients with HFpEF, for example, are more likely to be older, with a two-fold predominance of females. They also have a higher prevalence of the aforementioned non-cardiac comorbidities (hypertension, diabetes, stroke, etc.) that lead to chronic inflammation associated with HFpEF rather than HFrEF.
HFrEF is more common in men, which might be the result of their greater susceptibility to developing myocardial infarction (MI). Additionally, men more easily develop eccentric left ventricular hypertrophy upon pressure overload.
Diagnosis
Diagnosing HFpEF can be more difficult than diagnosing HFrEF. Diagnosing HFpEF relies upon the presence of symptoms and/or signs of heart failure, preserved left ventricular systolic function, and evidence of diastolic dysfunction.
Current diagnostic algorithms include clinical, laboratory, and instrumental characteristics; sophisticated imaging modalities; and invasive measurements. Many of these factors can be unclear and variable, which leads to diagnosis by exclusion, inconclusive diagnosis, or a missed diagnosis altogether.
Current diagnostic algorithms mainly rely on echocardiography (E/e’) and biomarkers (NT-proBNP). However, only a minority of patients with HFpEF are identified, and especially HFpEF patients at an early stage of the disease are easily missed.
We propose clinicians incorporate AI with echocardiography, which can be applied to cardiovascular imaging to greatly simplify the identification of patients with HFpEF. These additions to the current diagnostic work-up will improve diagnostic sensitivity and accurate staging of HFpEF patients.

In fact this AI technology has been proven to be 25% more accurate than current clinical algorithms, such as the H2FPEF and HFA-PEFF. Learn more
Researchers have additionally begun pooling knowledge across industries and disciplines to get a better understanding of HFpEF for a more accurate and timely diagnosis. This new initiative is called the Accelerating Medicines Partnership® Heart Failure (AMP® HF) Program, supported by the Foundation for the National Institutes of Health (FNIH). [8]


Treatment
Despite their significant differences in pathophysiology, treatment for both HFrEF and HFpEF has been largely similar. Current therapy is largely focused on the management of symptoms and comorbidities, such as medications to lower blood sugar in diabetic patients. Aside from lifestyle changes, physicians generally prescribe medications which reduce heart rate, lower blood pressure, reduce cholesterol levels and decrease the risk of blood clots.
In February 2022, The FDA approved the use of Eli Lilly and Boehringer Ingelheim's Jardiance (empagliflozin) for a broader range of patients with heart failure, including those with preserved ejection fraction (HFpEF), and shortly after, SGLT2is were added to the 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure.
The FDA clearance came after the EMPEROR-PRESERVED trial found the antidiabetic drug class sodium-glucose cotransporter-2 inhibitor (SGLT2i), specifically empagliflozin, showed that it can reduce cardiovascular death and hospitalizations in HFpEF, with a 29% lower risk of hospitalizations for heart failure.
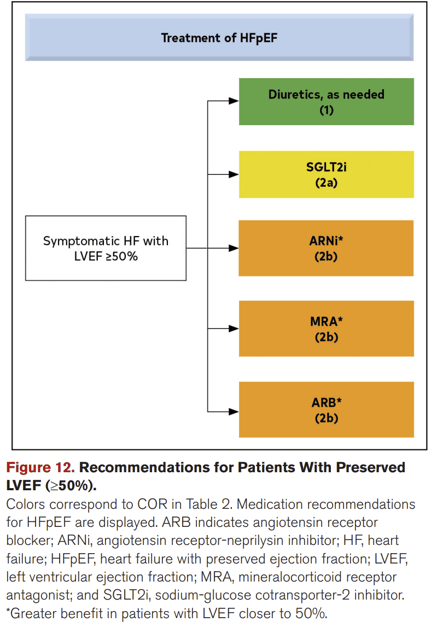
2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure
This new finding underscores the importance of precise and timely HFpEF diagnosis. [6,7]
The significant differences between development and presentation of HFpEF and HFrEF indicate how important it is to expand our knowledge of these conditions and how they manifest. Timely and precise diagnosis and treatment of HFpEF is still limited, but with new technologies, guidelines, and initiatives, the medical community is beginning to increase its understanding of the intricacies of this condition.
To learn more about HFpEF, including further details on diagnosing and risk stratifying the condition, click the link at the foot of the page.
References:
- Agosti F. Heart Failure in the United States [Internet]. The Cardiology Advisor. 2022.
- Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. European Heart Journal. 2010.
- American Heart Association. Ejection fraction heart failure measurement [Internet]. www.heart.org. American Heart Association; 2023.
- Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells. 2020 Jan 18;9(1):242.
- Heh SF, Phelan D, Abraham T, Armour A, Desai MY, Dragulescu A, et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. Journal of the American Society of Echocardiography. 2022;35:533–69.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. New England Journal of Medicine 2021;385:1451-1461
- American College of Cardiology. DELIVER: Dapagliflozin Lowered Risk of Worsening HF, Death in Patients With HFmrEF, HFpEF [Internet]. American College of Cardiology. 2022.
- Foundation for the National Institutes of Health. https://www.fnih.org/
Curious about upcoming research and innovation?
Sign up to hear about the latest news.



