
Automated Echocardiographic Detection Of HFpEF Using AI
- | By Ultromics
Ashley P. Akerman PhD* a, Mihaela Porumb PhD* a, Christopher G. Scott MSb, Arian Beqiri PhDa, Agisilaos Chartsias PhDa, Alexander J. Ryu MDc, William Hawkes PhDa, Geoffrey D. Huntley MDd, Ayana Z. Arystan MDd, Garvan C. Kane MDd, Sorin V. Pislaru MDd, Francisco Lopez-Jimenez MDd, Alberto Gomez PhDa, Rizwan Sarwar MRCPa,e,f, Jamie O’Driscoll PhDa,g, Paul Leeson MBa,e, Ross Upton PhDa, Gary Woodward PhDa, Patricia A. Pellikka MDd
Introduction
Detection of heart failure with preserved ejection fraction (HFpEF) involves integration of multiple imaging and clinical features which are often discordant or indeterminate. We hypothesized that applying artificial intelligence (AI) to the apical 4 chamber (A4C) echo videoclip, which contains a vast amount of information, might allow detection of HFpEF.
Aim
To develop an AI model to detect HFpEF from analysis of a single A4C transthoracic echocardiogram videoclip.
Methods
A three-dimensional convolutional neural network was developed and trained on A4C videoclips to classify patients with HFpEF (diagnosis of HF, EF≥50%, and echocardiographic evidence of increased filling pressure; cases) versus without HFpEF (EF≥50%, no diagnosis of HF, normal filling pressure; controls).
Model outputs were classified as HFpEF, no HFpEF, or nondiagnostic (high uncertainty). Performance was assessed in an independent multi-site dataset and compared to the previously validated clinical scores, HFA-PEFF and H2FPEF. For the test group, mortality was evaluated using the Kaplan-Meier method, censoring subjects at last follow-up. Survival curves and Cox proportional hazards regression estimate of hazard ratio were adjusted for age.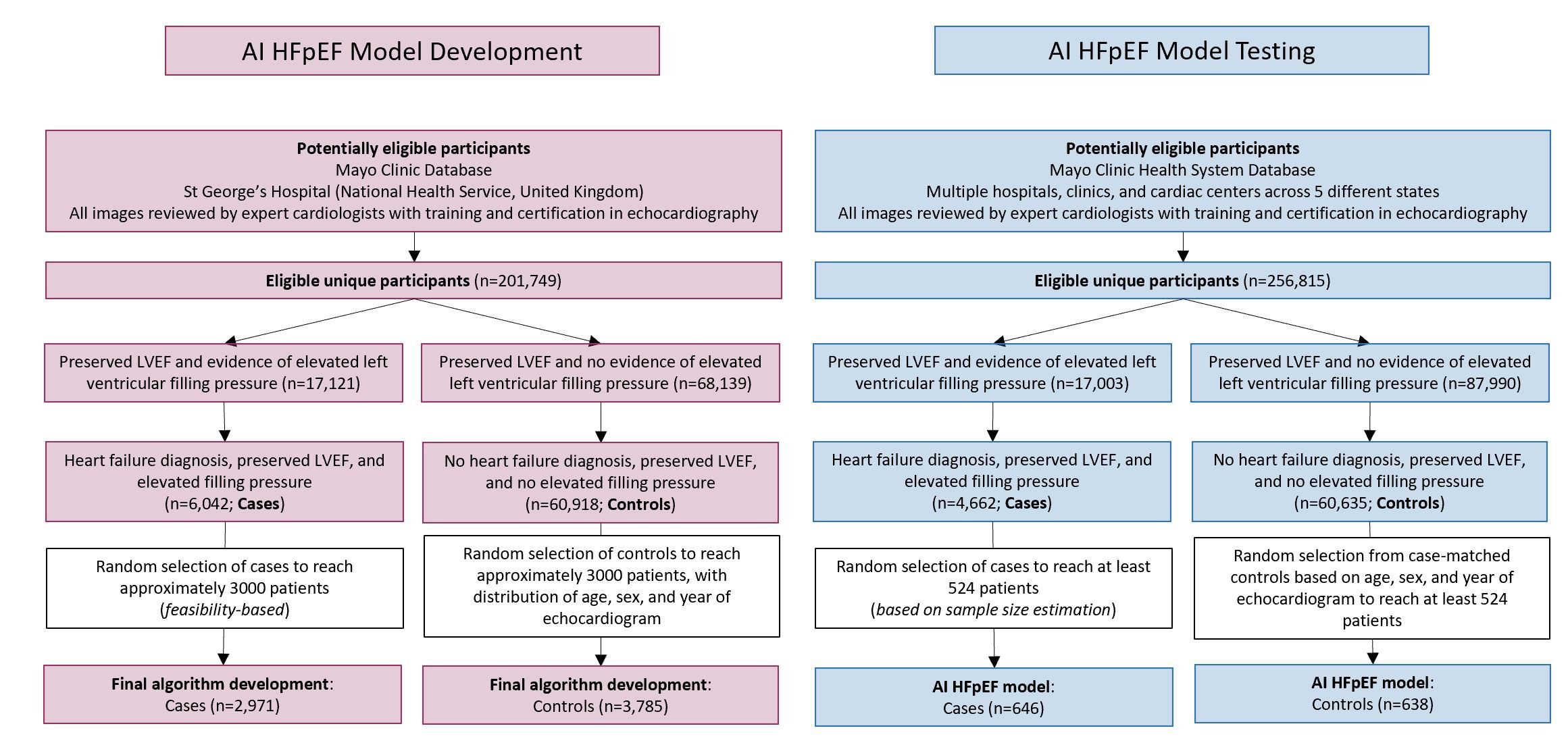
Results
Training and validation included 2971 cases and 3785 controls (validation holdout, 16.8% patients), and demonstrated excellent discrimination (AUROC:0.97 [95%CI:0.96-0.97] and 0.95 [0.93-0.96] in training and validation, respectively).
In independent testing (646 cases, 638 controls), 94 (7.3%) were non-diagnostic; sensitivity (87.8%; 84.5-90.9%) and specificity (81.9%; 78.2-85.6%) were maintained in clinically relevant subgroups, with high repeatability and reproducibility.
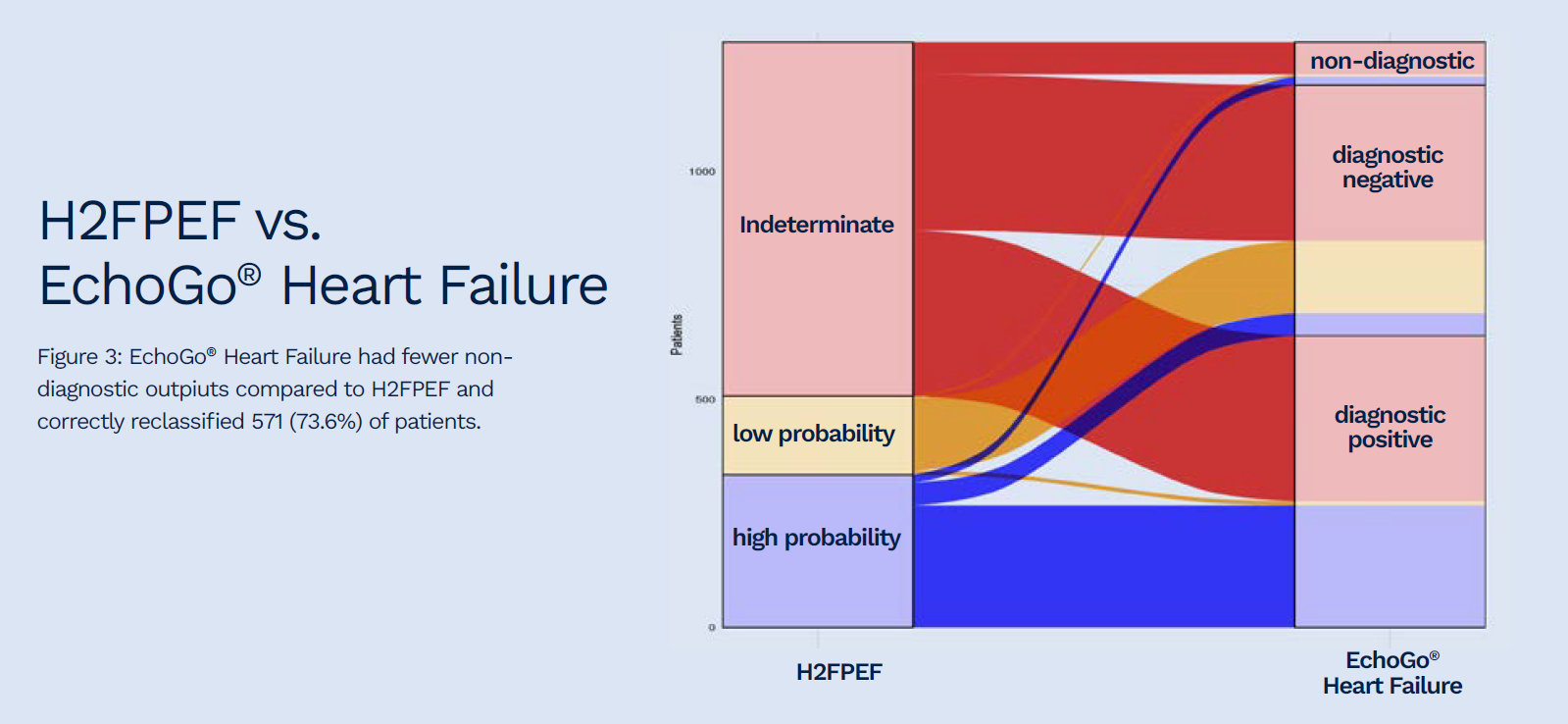
Of 701 and 776 indeterminate outputs from the HFA-PEFF and H2FPEF scores, the AI HFpEF model correctly reclassified 73.5 and 73.6%, respectively.
During follow-up (median [IQR]:2.3 [0.5-5.6] years), 444 (34.6%) patients in the test group died; mortality was higher in patients classified as HFpEF by AI (hazard ratio [95%CI]:1.9[1.5-2.4]).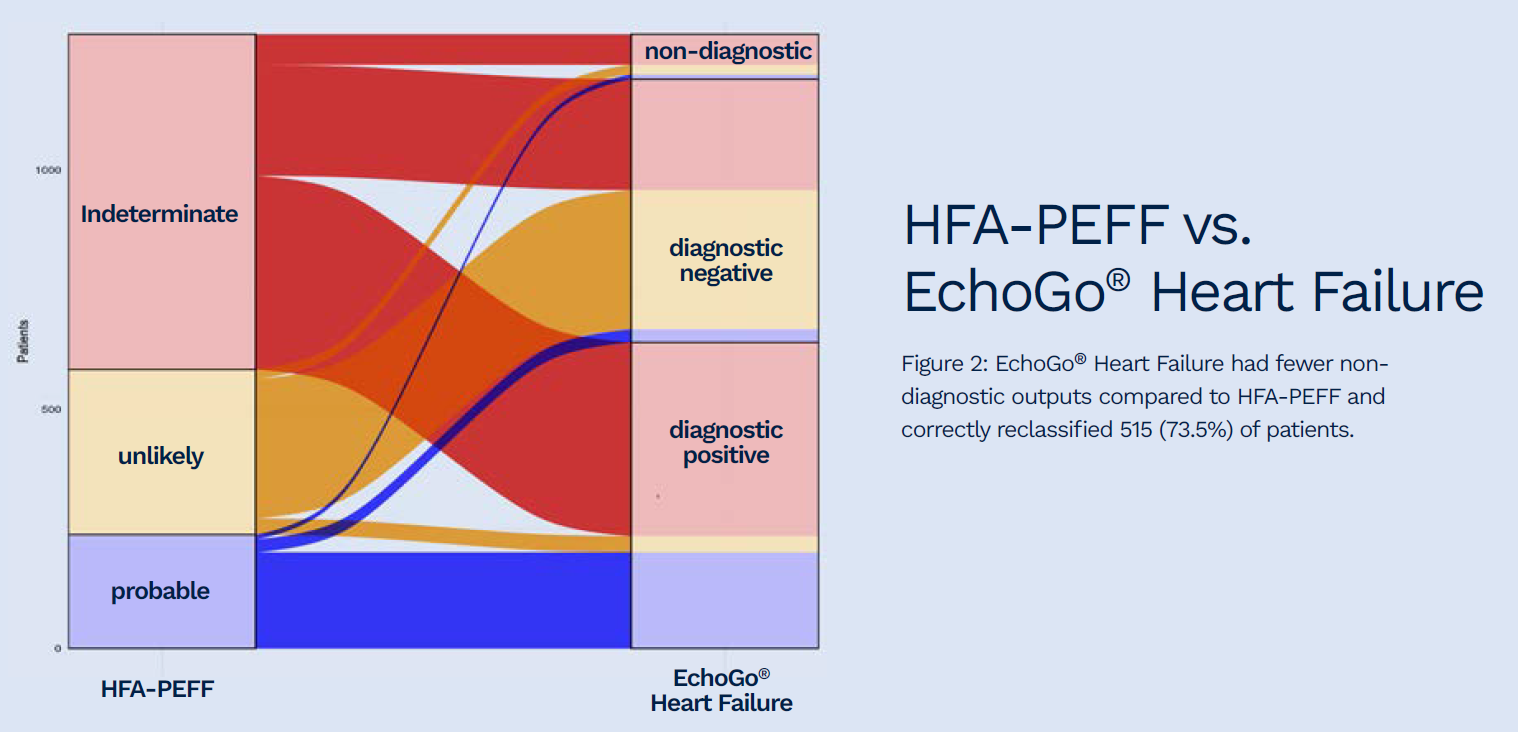
Limitations
It is possible that some controls had subclinical disease. Complete matching for age was not possible; patients with HFpEF for older. However, survival analysis was age adjusted and sensitivity analysis demonstrated no meaningful change in interpretation in a subgroup of agematched patients.
Conclusion
This novel AI HFpEF model based on a single, routinely acquired echocardiographic video demonstrated excellent discrimination of patients with versus without HFpEF, more often than clinical scores, and identified patients with higher mortality.
The application of this classifier in the screening for HFpEF, particularly when their diagnosis is uncertain, has the potential to automate an accurate detection process for a complex clinical syndrome, resulting in more patients getting a correct and expeditious diagnosis.
